mRNA Quality Monitoring Market to Reach USD 2.5 Billion by 2034, Driven by Expanding Healthcare Applications
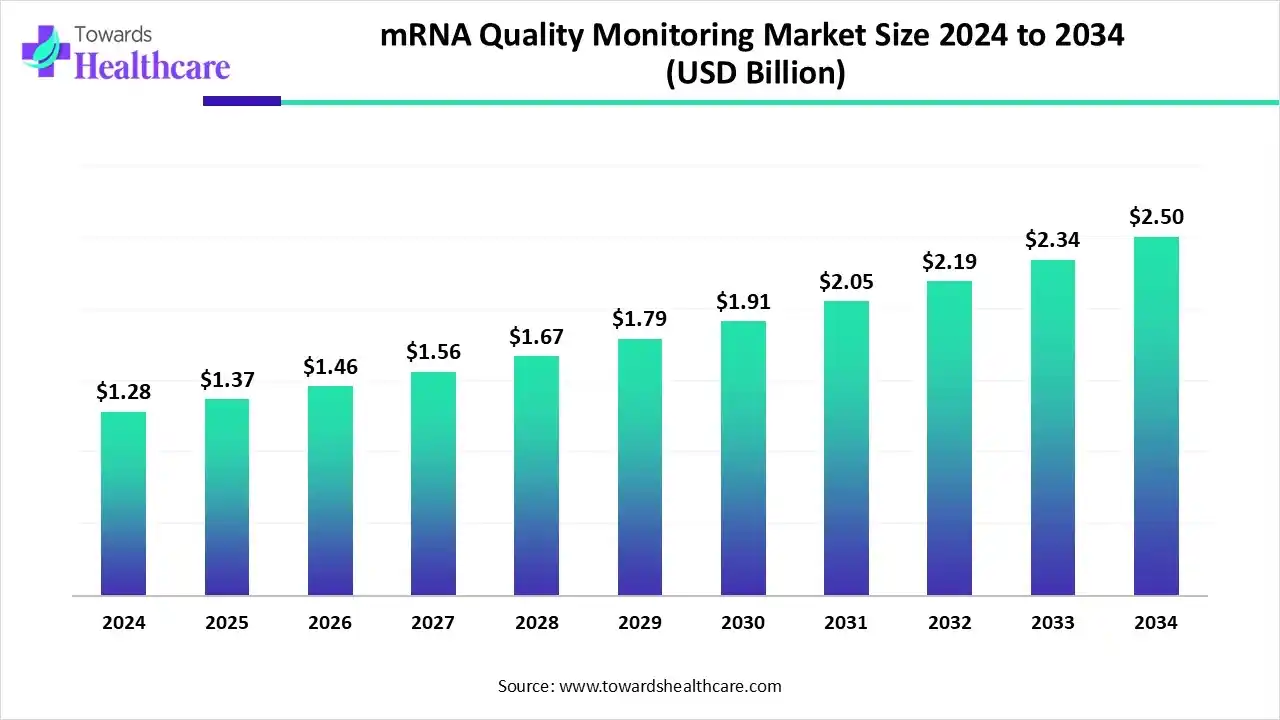
The global mRNA quality monitoring market size was valued at USD 1.28 billion in 2024 and is predicted to hit around USD 2.5 billion by 2034, rising at a 6.97% CAGR, a study published by Towards Healthcare a sister firm of Precedence Research.
Ottawa, Dec. 11, 2025 (GLOBE NEWSWIRE) -- The global mRNA quality monitoring market size is calculated at USD 1.37 billion in 2025 and is expected to reach around USD 2.5 billion by 2034, growing at a CAGR of 6.97% for the forecasted period, driven by the expanding healthcare applications and stringent regulations.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report @ https://www.towardshealthcare.com/download-sample/6400
Key Takeaways
- North America held a major revenue share of the market in 2025.
- Asia Pacific is expected to witness the fastest growth in the mRNA quality monitoring market during the forecast period.
- By product & service, the kits, assays & reagents segment held a major revenue share of the market in 2025.
- By product & service, the instruments & software segment is expected to witness the fastest growth in the market during the forecast period.
- By technology, the electrophoresis technologies segment held a major revenue share of the market in 2025.
- By technology, the advanced/emerging technologies segment is expected to witness the fastest growth in the market during the forecast period.
- By end-use, the biopharmaceutical & biotechnology companies segment held a major revenue share of the market in 2025.
- By end-use, the CDMOs/CROs & analytical service providers segment is expected to witness the fastest growth in the market during the forecast period.
- By application area, the vaccine development & commercial manufacturing segment held a major revenue share of the market in 2025.
- By application area, the therapeutic mRNA segment is expected to witness the fastest growth in the market during the forecast period.
What is Meant by mRNA Quality Monitoring?
The mRNA quality monitoring market is driven by increasing R&D investment, stringent regulations, and the adoption of mRNA-based therapeutics and vaccines. mRNA quality monitoring refers to the testing of the quality, consistency, and integrity of messenger RNA molecules during their R&D, or development of vaccines and therapeutics. They are used for the assessment of size, purity, sequence fidelity, encapsulation, and stability.
What are the Major Growth Drivers in the mRNA Quality Monitoring Market?
The growing demand for personalized medicines is the major driver in the market. This is increasing the development of mRNA therapeutics, which is driving the demand for their quality monitoring solutions to ensure their safety and efficacy. Additionally, demand for sequence fidelity, outsourcing trends, and the adoption of advanced technologies are other market drivers.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
What are the Key Drifts in the mRNA Quality Monitoring Market?
The market has been expanding due to the growing funding to launch and enhance the use of various mRNA quality monitoring products and services.
- In March 2025, a total of $4,500,000 was secured by Intelliseq, where the funding will be used to advance next-generation sequencing platforms, computational infrastructure, and expand the service portfolio in the field of genomics.
- In January 2025, $30 million in a Series D financing round was raised by Clear Labs, which will be utilized to enhance the development and adoption of its cutting-edge NGS solutions.
What is the Significant Challenge in the mRNA Quality Monitoring Market?
High cost acts as the major challenge in the market. The instruments and technologies are expensive, where the repeated use of reagents and consumables also adds up to the cost, which limits their use. Moreover, complex workflows, shortage of skilled personnel, and batch-to-batch variability are other market restraints.
Regional Analysis
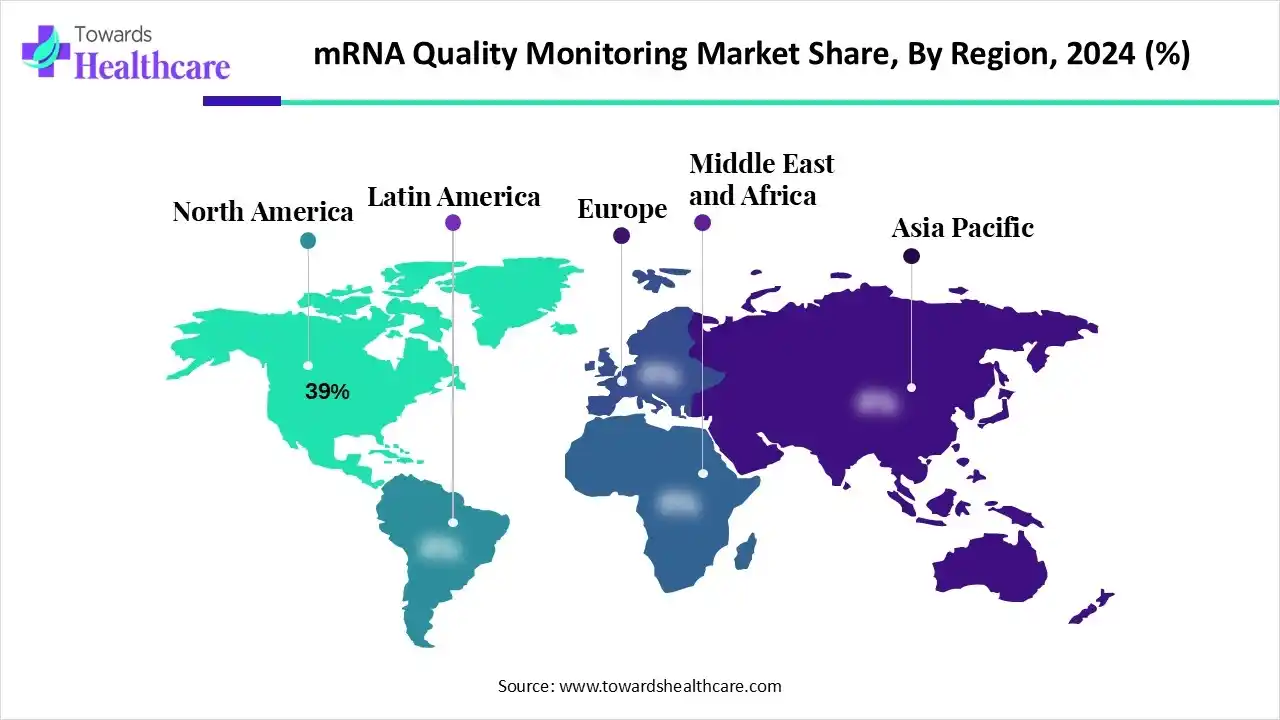
Why did North America Dominate the mRNA Quality Monitoring Market in 2025?
In 2025, North America captured the biggest revenue share in the market, due to the presence of robust industries. This, in turn, increased the production of various mRNA products, which increased the use of their quality monitoring solutions. The stringent regulation and R&D investments also increased their innovation and adoption rates, which contributed to the market growth.
What Made Asia Pacific the Fastest Growing region in the mRNA Quality Monitoring Market in 2025?
Asia Pacific is expected to be the fastest-growing region in the market during the forecast period, due to expanding industries. This increased the development and innovation of mRNA therapeutics, which increased their clinical trials as well, driving the demand for mRNA quality continuing solutions. Additionally, government funding, investments, and expanding the CDMO ecosystem are also increasing their use, promoting market growth.
Become a valued research partner with us - https://www.towardshealthcare.com/schedule-meeting
Segmental Insights
By product & service analysis
Why Did the Kits, Assays & Reagents Segment Dominate in the mRNA Quality Monitoring Market in 2025?
By product & service, the kits, assays & reagents segment led the market in 2025, as they were used repeatedly for quality checks. They were also used for the development of ready-to-use kits, which were used to overcome the complex QC workflows. They were also used for a wide range of applications.
By product & service, the instruments & software segment is expected to show the fastest growth rate in the market during the predicted time, due to the growing use of automated QC instruments. Advanced technologies are also being used for sequence analysis and process optimization.
By technology analysis
Which Technology Type Segment Held the Dominating Share of the mRNA Quality Monitoring Market in 2025?
By technology, the electrophoresis technologies segment held the dominating share of the market in 2025, driven by wide acceptance rates. They were used for R&D and manufacturing workflows. At the same time, their affordability and integration with advanced technologies also increased their use.
By technology, the advanced/emerging technologies segment is expected to witness the fastest growth in the market during the predicted time, due to growing demand for enhanced precision. They are also providing real-time monitoring and automation, which is increasing their adoption rates.
In the advanced/emerging technologies segment, the NGS (Next-Generation Sequencing) for sequence fidelity subsegment is expected to show the highest growth during the predicted time, driven by their enhanced accuracy and impurity detection. This is increasing their use in mRNA vaccines and therapeutic development.
By end-user analysis
How the Biopharmaceutical & Biotechnology Companies Segment Dominated the mRNA Quality Monitoring Market in 2025?
By end-use, the biopharmaceutical & biotechnology companies segment led the market in 2025, driven by their large-scale manufacturing of mRNA products. This, in turn, increased the demand for their quality, safety, and purity testing. Moreover, the R&D investments also promoted their adoption.
By end-use, the CDMOs/CROs & analytical service providers segment is expected to show the fastest growth rate in the market during the upcoming years, due to growing outsourcing trends. Similarly, the growing demand for specialized experts and increasing advancements in mRNA therapeutics are also increasing their demand.
By application area analysis
What Made Vaccine Development & Commercial Manufacturing the Dominant Segment in the mRNA Quality Monitoring Market in 2025?
By application area, the vaccine development & commercial manufacturing segment held the largest share of the market in 2025, due to its high production volume. At the same time, the stringent regulations and complex workflows also increased the demand for mRNA quality monitoring solutions.
By application area, the therapeutic mRNA segment is expected to show the highest growth in the market during the upcoming years, due to growing interest in chronic diseases. This is driving the development of mRNA therapeutics, where their complex production and demand for personalized medications are also increasing the use of mRNA quality monitoring solutions.
Get the latest insights on life science industry segmentation with our Annual Membership: https://www.towardshealthcare.com/get-an-annual-membership
Recent Developments in the mRNA Quality Monitoring Market
- In July 2025, four new Droplet Digital™ PCR (ddPCR™) platforms were launched by Bio-Rad Laboratories, Inc., which will support the company’s absolute quantification, advanced multiplexing capabilities, and high precision, and will simplify their workflows.
- In February 2025, a proprietary, breakthrough sequencing by expansion (SBX) technology, providing ultra-rapid and high-throughput sequencing, was launched by Roche.
mRNA Quality Monitoring Market Key Players List
- QIAGEN N.V.
- Revvity, Inc.
- Illumina, Inc.
- Sartorius AG
- Charles River Laboratories
- Eurofins Scientific SE
- TriLink BioTechnologies (Maravai LifeSciences)
- Creative Biolabs
- GenScript Biotech Corporation
- Vazyme Biotech Co., Ltd.
Browse More Insights of Towards Healthcare:
The global mRNA therapeutics CDMO market size is calculated at USD 4.62 billion in 2024, grew to USD 5.15 billion in 2025, and is projected to reach around USD 13.63 billion by 2034. The market is expanding at a CAGR of 11.37% between 2025 and 2034.
The consumption-type vaccine market is on an upward trajectory, poised to generate substantial revenue growth, potentially climbing into the hundreds of millions over the forecast years from 2025 to 2034.
The injection flu shot market size stood at US$ 10.63 billion in 2024, grew to US$ 11.8 billion in 2025, and is forecast to reach US$ 29.97 billion by 2034, expanding at a CAGR of 10.93% from 2025 to 2034.
The global RNA analysis market size is calculated at US$ 6.86 billion in 2025, grew to US$ 7.78 billion in 2026, and is projected to reach around US$ 23.9 billion by 2035. The market is expanding at a CAGR of 13.36% between 2025 and 2034.
The global RNA based therapeutic market was evaluated at US$ 6.83 billion in 2023 and is expected to attain around US$ 40.81 billion by 2034, growing at a CAGR of 17.64% from 2024 to 2034.
The RNA therapeutics and vaccines market size is estimated at US$ 0.21 billion in 2024, is projected to grow to US$ 0.38 billion in 2025, and is expected to reach around US$ 107.7 billion by 2034, at a CAGR of 87% between 2025 and 2034.
The RNAi therapeutics market is forecast to grow at a CAGR of 14.9%, from USD 1.47 billion in 2025 to USD 5.11 billion by 2034, over the forecast period from 2025 to 2034.
The global NGS-based RNA-sequencing market size is calculated at USD 3.74 billion in 2024, grow to USD 4.49 billion in 2025, and is projected to reach around USD 23.52 billion by 2034. The market is expanding at a CAGR of 20.1% between 2025 and 2034.
The global miRNA sequencing and assay market size is calculated at US$ 391.73 in 2024, grew to US$ 443.95 million in 2025, and is projected to reach around US$ 1369.11 million by 2034. The market is expanding at a CAGR of 13.33% between 2024 and 2034.
The global microRNA market was estimated at US$ 2.31 billion in 2026 and is projected to grow to US$ 6.89 billion by 2035, rising at a compound annual growth rate (CAGR) of 12.89% from 2026 to 2035.
Segments Covered in The Report
By Product & Service
- Kits, Assays & Reagents
- Integrity assays (capillary electrophoresis, fragment analysis)
- Purity assays (HPLC, LC-MS, UV-Vis quantification)
- Endotoxin & dsRNA detection reagents
- Instruments & Software
- Electrophoresis systems (capillary, gel, microfluidic)
- LC-MS & chromatography systems
- Software for data analytics, traceability & automation
- QC Testing & Analytical Services
- Contract testing
- GMP release & stability studies
- Biosafety & regulatory documentation services
By Technology
- Electrophoresis Technologies
- Capillary electrophoresis (CE)
- Microfluidic chip-based electrophoresis
- Liquid Chromatography & Mass Spectrometry (LC-MS)
- LC-MS for molecular weight & purity profiling
- UHPLC for small impurity detection
- PCR-based Methods
- qPCR for sequence verification
- RT-PCR for residual DNA quantification
- ELISA/Immunoassays
- dsRNA detection
- capping enzyme quantification
- Advanced/Emerging Technologies
- NGS (Next-Generation Sequencing) for sequence fidelity
- CRISPR-based QC for transcript validation
By End-Use
- Biopharmaceutical & Biotechnology Companies
- mRNA vaccine developers
- Therapeutic mRNA producers
- CDMOs/CROs & Analytical Service Providers
- Contract QC & process validation labs
- GMP lot release & stability testing
- Academic & Research Institutes
- Pre-clinical characterization
- Process optimization & method development
By Application Area
- Vaccine Development & Commercial Manufacturing
- Therapeutic mRNA
- Gene Editing & CRISPR mRNA QC
- Process Development & Validation
- Regulatory Release & Stability Testing
By Region
North America
- U.S.
- Canada
- Mexico
- Rest of North America
South America
- Brazil
- Argentina
- Rest of South America
Europe
-
Western Europe
- Germany
- Italy
- France
- Netherlands
- Spain
- Portugal
- Belgium
- Ireland
- UK
- Iceland
- Switzerland
- Poland
- Rest of Western Europe
- Eastern Europe
- Austria
- Russia & Belarus
- Türkiye
- Albania
- Rest of Eastern Europe
Asia Pacific
- China
- Taiwan
- India
- Japan
- Australia and New Zealand,
- ASEAN Countries (Singapore, Malaysia)
- South Korea
- Rest of APAC
MEA
- GCC Countries
- Saudi Arabia
- United Arab Emirates (UAE)
- Qatar
- Kuwait
- Oman
- Bahrain
- South Africa
- Egypt
- Rest of MEA
Immediate Delivery Available | Buy This Premium Research @ https://www.towardshealthcare.com/checkout/6400
Access our exclusive, data-rich dashboard dedicated to the healthcare market - built specifically for decision-makers, strategists, and industry leaders. The dashboard features comprehensive statistical data, segment-wise market breakdowns, regional performance shares, detailed company profiles, annual updates, and much more. From market sizing to competitive intelligence, this powerful tool is one-stop solution to your gateway.
Access the Dashboard: https://www.towardshealthcare.com/access-dashboard
About Us
Towards Healthcare is a leading global provider of technological solutions, clinical research services, and advanced analytics, with a strong emphasis on life science research. Dedicated to advancing innovation in the life sciences sector, we build strategic partnerships that generate actionable insights and transformative breakthroughs. As a global strategy consulting firm, we empower life science leaders to gain a competitive edge, drive research excellence, and accelerate sustainable growth.
You can place an order or ask any questions, please feel free to contact us at sales@towardshealthcare.com
Europe Region: +44 778 256 0738
North America Region: +1 8044 4193 44
APAC Region: +91 9356 9282 04
Web: https://www.towardshealthcare.com
Our Trusted Data Partners
Precedence Research | Statifacts | Towards Packaging | Towards Automotive | Towards Food and Beverages | Towards Chemical and Materials | Towards Consumer Goods | Towards Dental | Towards EV Solutions | Nova One Advisor | Healthcare Webwire | Packaging Webwire | Automotive Webwire | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant | Specialty Chemicals Analytics
Find us on social platforms: LinkedIn | Twitter | Instagram | Medium | Pinterest

Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.

